
October 23, 2018
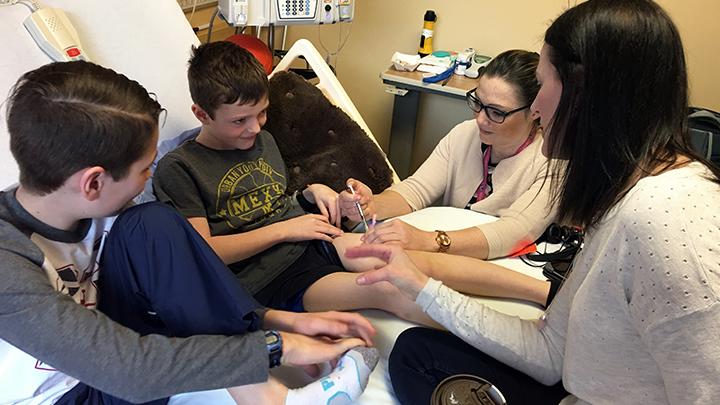
It’s a big moment for Jace Owen, as Stollery nurse Heather Bauman gives him his very first injection to treat his severe Hemophilia A. Prior to this treatment, Jace had daily IV treatments that were beginning to lose their effect. Since his first Hemlibra injection on April 11, Jace only requires one injection per week, which has finally allowed him to play like other children his age.
Story by Vanessa Gomez
EDMONTON — Most children lead very active lives with sports and activities, with little worry of injury.
But reality is very different for eight-year-old Jace Owen, who lives with Hemophilia A, a condition that calls for extra caution. He lacks a major blood-clotting protein that puts him at risk of severe and prolonged bleeds, and prevents him from participating in many activities typical to children his age.
Diagnosed with this rare genetic disorder at birth, Jace’s journey was further complicated by inhibitors, antibodies which inactivate conventional hemophilia treatments. Since the age of sixteen months he’s been getting daily injections through an Intravenous Vascular Access Device (IVAD). As a result of the inhibitors, severe bleeds require up to four or five injections per day and even these treatments may not stop the bleeding, leaving him with few options for relief.
“Over the past few years, he has been on many medications,” says his mother, Leanne Owen. “When his medication wasn’t working he could not participate in gym class or recess, as even using the monkey bars put him at high risk for joint bleeds.”
After jumping off his bed, which resulted in severe bleeding in his ankle joint and led to a bone infection, Jace required immediate hospitalization. He proved unresponsive to available treatments, leaving his family and care team desperate for a new avenue of care.
Luckily, Leanne had been following a new treatment, Hemlibra by Roche, the Swiss biotech company. It’s the first new treatment in 20 years for patients with severe Hemophilia A.
Since the treatment had yet to be approved in Canada, Jace was initially denied access to it — but as his condition worsened, Hemlibra soon became his last option.
After copious amounts of paperwork — and with Health Canada, the Canadian Hemophilia Society and Roche fighting alongside Jace’s care team to give him a chance — Jace became the first child in Canada to be approved for Hemlibra.
“It was scary and exciting because none of the conventional treatments would work,” says Heather Bauman, a pediatric hemophilia and bleeding disorders nurse at the Stollery Children’s Hospital. "It was such a relief that we had another line of treatment.”
“I was fairly confident it would work,” says Dr. Mark Belletrutti, a pediatric hematologist at the Stollery. “We knew from other research that it worked quite well in other patients.”
Since Jace received his first injection on April 11 — and a once-a-week injection since then — he has not had a bleed.
Hemlibra was approved in Canada on Aug. 3. While it’s only available for Hemophilia A with inhibitors, Dr. Belletrutti is confident it will open up doors for the whole hemophilia community.
“It’s giving everyone a second look as to who is able to go on this medication when it becomes available. Who are the ideal patients? What restrictions are we going to have?” he adds.
With Hemlibra, Jace says he enjoyed this past summer much like other kids — camping with his family, coast jumping, downhill mountain biking and playing on the monkey bars.
“We’re cautiously optimistic,” says Owen. “We know he’s protected right now and hope that continues”