
July 14, 2015
Story by Gregory Kennedy; photo by Jason Franson
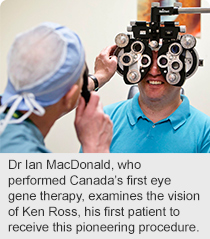
Over the years the light has been fading to black for Ken Ross, who was born with choroideremia, a form of inherited blindness that leaves those affected blind by middle age.
Now 43, with only five per cent vision remaining in his right eye, the things most of us take for granted, such as looking up to see the stars in the night sky, is now pretty much a memory for the 43-year-old Edmontonian.
But for the first time in his life — as one of six men and the first person in Canada to undergo Canada’s first human gene therapy trial for eyes — Ross now looks forward to a potentially clearer, brighter future.
“I feel like I won the lottery — being the first to get this treatment,” he says with emotion. “I’m still trying to wrap my head around it.”
 “I didn’t foresee this happening in my lifetime. I’ll try not to cry. I’ve been seeing Dr. MacDonald since I was little. I got tested, hoping to take part in the trial, last August. Then my spouse and I went on a cruise. I was sitting on a ship’s balcony thinking, ‘This is just my lot in life. I’ll enjoy the view while I can and keep the memory inside.’ And when I came back, I got the call that I qualified for the surgery.”
“I didn’t foresee this happening in my lifetime. I’ll try not to cry. I’ve been seeing Dr. MacDonald since I was little. I got tested, hoping to take part in the trial, last August. Then my spouse and I went on a cruise. I was sitting on a ship’s balcony thinking, ‘This is just my lot in life. I’ll enjoy the view while I can and keep the memory inside.’ And when I came back, I got the call that I qualified for the surgery.”
Gene therapy involves the replacement of a faulty gene with a healthy one. The clinical trial now underway at the Royal Alexandra Hospital aims to preserve and potentially restore vision for people with this genetic disorder.
“It’s a great privilege to be able to do something very positive for people with choroideremia,” says clinical research team leader Dr. Ian MacDonald, an ophthalmologist with Alberta Health Services and professor with the Faculty of Medicine & Dentistry at the University of Alberta, sponsor of the research. “People have hoped for this for a long time.”
Choroideremia is a form of inherited blindness that affects one in 50,000 people, about 90 per cent of whom are men. Many experience difficulty seeing at night during their teens, lose peripheral vision in adulthood and are often legally blind by the age of 40. The disease is caused by a faulty gene that results in the degeneration of the light-sensing retinal cells at the back of the eye. Until now, it has been untreatable.
Gene therapy is not a drug but rather a transfer of human genes. Gene therapy refers to the incorporation of new DNA into cells, either to replace a gene that is missing or not functioning. This allows the cells to produce an important protein. In choroideremia, this protein is not produced and retinal cells die off over time, causing vision to deteriorate.
Dr. MacDonald’s trial involves a new treatment intended to stop choroideremia in its tracks with a single injection of what’s known as a viral vector — a small harmless virus that’s been modified to carry into the eye the ‘good’ gene needed to potentially prevent further loss of sight and to restore the vision of his patients — often with noticeable results in under a month.
During the procedure, performed under a general anesthetic, a surgeon detaches the area to be injected in the patient’s retina, then injects the viral vector through a narrow needle into the back of the eye. The injection carries about 10 billion viral particles, each carrying a working copy of the good CHM gene, to target millions of eye cells.
“We’re using the natural properties of a virus, its ability to inject its DNA into human cells, to introduce a working copy of the needed gene into the retinal layers,” says Stephanie Chan, genetic counsellor and study co-ordinator. “The idea is that those cells will take up the good gene and use it to create the correct protein, replacing the defective gene that’s in the cells.”
The viral vector, known as AAV2-REP1, was provided by NightstaRx Ltd., a private British biopharmaceutical company focused on the development of therapies for retinal dystrophies.
“We are leading the way in the development of an effective gene therapy treatment for choroideremia and this new study, sponsored by the University of Alberta, is another step forward in the development of AAV2-REP1,” says David Fellows, CEO of NightstaRx. “We have been granted Orphan Drug Designation for the product in the United States and Europe and the data to date has shown very promising results.”
The first clinical trials took place at the University of Oxford. Results published in The Lancet Medical Journal in 2014 reported that six months after treatment, the first six patients showed improvement in their vision in dim light; and two of the six were able to read more lines on an eye chart. Oxford research is ongoing under the direction of ophthalmologist Dr. Robert MacLaren.
Funds, support and equipment for the Canadian trial have been provided by various governmental and private agencies, including Alberta Innovates – Health Solutions (AIHS); Canadian Institutes of Health Research (CIHR); NightstaRx; Canada Foundation for Innovation; Alberta Innovates (Alberta Innovation and Advanced Education); The Foundation Fighting Blindness (FFB); Choroideremia Research Foundation Canada (CRFC); and private donors.
Researchers believe this new approach to eye therapy has promise for treating people early on before too many cells in the retina have been lost. It may also have relevance for other, far more common causes of blindness, such as retinitis pigmentosa and age-related macular degeneration.
“If we can maintain the vision our patients have, we would look at that as a success,” says Chan. “If there is improvement, that’s even better.”
Dr. MacDonald says he’s “absolutely impressed” by the eye’s ability to heal itself and reattach the retina within hours of surgery. “The human body is doing its work. We’re just helping it.”
Barely five weeks after Ross underwent his procedure on May 25, he says his vision continues to improve.
“I was outside gardening on the weekend, and I noticed how colours look so much more vibrant now,” he says.
“Today at work, I was able to read the bold type in the subject line of my emails using only my right eye — and I haven’t been able to do that in years.”